Abstract
Background: Over 13,000 hematopoietic stem cell transplants (HSCT) are performed each year to treat malignant and non-malignant hematologic diseases in the United States. Infections are among the leading causes of mortality after HSCT and vaccination is one of the most important preventive strategies available for infectious diseases. The Infectious Disease Society of America (IDSA) has guidelines regarding re-vaccination, starting as early as 6 months post-HSCT based on the type of HSCT and clinical condition of the patient. There are however limited available data on adherence with these guidelines. As such the immune dysregulation and pathobiology working group report of the NIH HSCT late-effects initiative identified the study of the adherence with consensus guidelines for post-HSCT as a key research recommendation.
Objective: To determine the adherence for all recommended vaccines based on the 2013 IDSA guidelines up to 2 years post-HSCT in a retrospective study at a large pediatric HSCT program
Methods: A retrospective chart review using electronic medical records (EMR) of all patients who have undergone HSCT or have received post-HSCT care at Children's Healthcare of Atlanta (CHOA) from January 2010 to December 2016. We collected demographic information (age, gender, race, and socioeconomic status) as well as the date of HSCT, date of last clinic visit, and date of vaccine administration. We reviewed vaccinations and dates administered using the Georgia Registry of Immunization Transactions and Services (GRITS), and determined if these were given per the IDSA guidelines. Vaccines were administered according to standardized protocol and recommendation communicated by HSCT physician to the referring oncologist and primary care physicians except for pneumococcal vaccines and meningococcal vaccines in patients with sickle cell disease (SCD) which are administered in the HSCT clinic during follow up in the multidisciplinary " Ex-Sickle" late-effects clinic. We compared vaccination rates in SCD post-HSCT to vaccination rates for other indications. We used univariate logistic regression to identify risk factors for low compliance. Our final model included age group, gender, and health insurance coverage and clinic type. Values of p <0.05 were considered statistically significant. The analyses were carried out using R version 3.4.2.
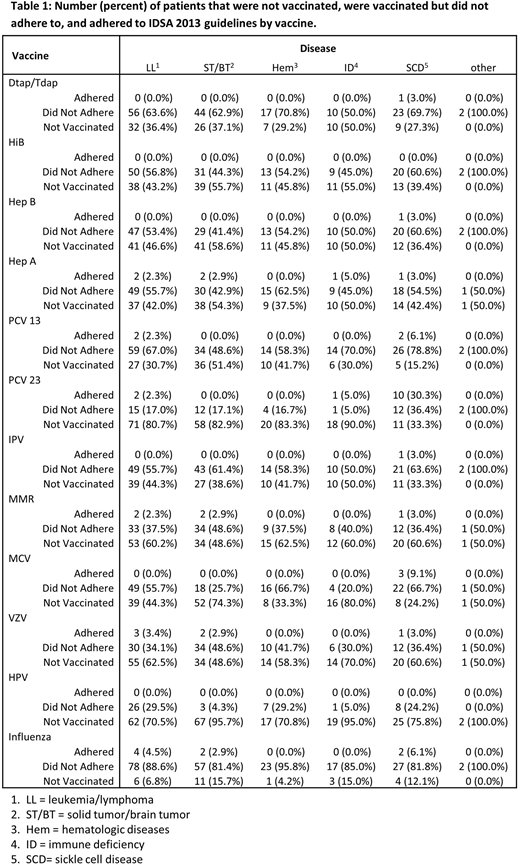
Results: Of 237 patients surviving at least two years post-HSCT, greater than 94% of patients did not receive one or more vaccines in accordance with the 2013 IDSA guidelines. Table 1 provides the detailed disease specific breakup of vaccine adherence. Given the small sample sizes for vaccine adherence, the remainder of our results focus on whether patients received any of doses of the recommended vaccinations, regardless of time since HSCT. SCD patients had significantly higher odds of receiving pneumococcal polyscaaharide vaccine (PPV23) (OR: 0.12; 95% CI: 0.05, 0.29) and meningococcal vaccine (MCV )(OR: 0.40; 95% CI: 0.16, 0.96) compared to patients with Leukemia/Lymphoma (LL), higher odds of receiving Hep B (OR: 0.40; 95% CI: 0.17, 0.94 ), Pneumococcal conjugate vaccines (PCV 13) (OR: 0.17; 95% CI: 0.05, 0.45), PPV 23 (OR: 0.10; 95% CI: 0.04, 0.26), MCV (OR: 0.11; 95% CI: 0.04, 0.28), and HPV (OR: 0.14; 95% CI: 0.03, 0.53) vaccines relative to patients with Solid tumor/Brain tumor (ST/BT), and significantly higher odds of receiving PCV 13 (OR: 0.25; 95% CI: 0.07, 0.84) and PPV 23 (OR: 0.10; 95% CI: 0.02, 0.34) relative to patients with other hematologic diseases (Hem), and SCD patients had significantly higher odds of receiving PPV 23 (OR: 0.06; 95% CI: 0.01, 0.24) and MCV (OR: 0.08 ; 95% CI: 0.02, 0.29) vaccines relative to the patients with Immune Deficiency (ID).
Discussion: These data have uncovered a major potential gap in service delivery in a large pediatric transplant program. SCD patients had higher rates of adherence with vaccination post-HSCT suggests the benefit of follow up in a multidisciplinary late-effects clinic. Further studies are needed to identify the factors impacting the vaccine adherence in order to increase the compliance to improve outcomes among HSCT patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal